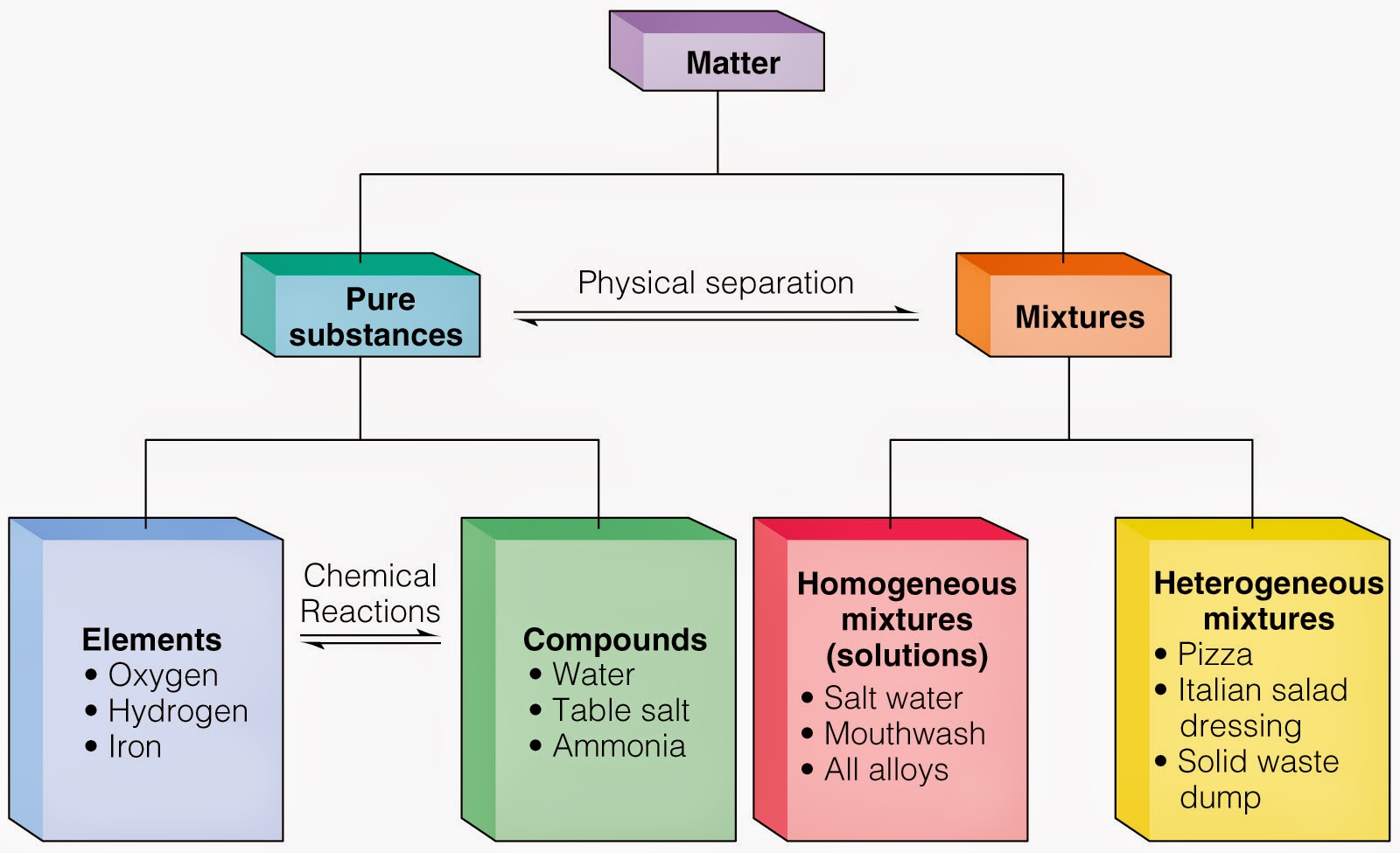
Is Vinegar An Element A Compound Or A Mixture . The molecular formula for water is h 2 o. If its composition is uniform throughout, it is a homogeneous mixture. Italian dressing is an example of. vinegar consists of acetic acid (ch 3 cooh), water, and trace amounts of other chemicals, which may include flavorings. The structural formula for acetic acid is ch 3 cooh. If a substance is not chemically pure, it is either a heterogeneous mixture or a homogeneous mixture. vinegar is not considered a compound, as it is a mixture of acetic acid and water that can be physically separated. if a substance can be separated into its elements, it is a compound. It is a mixture that includes substances other than acetic acid and water. a mixture with a composition that varies from point to point is called a heterogeneous mixture. The concentration of the acetic acid is variable. in this sense, vinegar can be considered a compound due to the acetic acid it contains. no, vinegar is not a pure compound. So, there are actually two main chemical formulas involved. On the other hand, vinegar can also be.
from circuitdiagramheure.z21.web.core.windows.net
no, vinegar is not a pure compound. So, there are actually two main chemical formulas involved. Italian dressing is an example of. The molecular formula for water is h 2 o. in this sense, vinegar can be considered a compound due to the acetic acid it contains. if a substance can be separated into its elements, it is a compound. On the other hand, vinegar can also be. It is a mixture that includes substances other than acetic acid and water. If a substance is not chemically pure, it is either a heterogeneous mixture or a homogeneous mixture. vinegar consists of acetic acid (ch 3 cooh), water, and trace amounts of other chemicals, which may include flavorings.
Mixture Of An Element And A Compound Diagram
Is Vinegar An Element A Compound Or A Mixture no, vinegar is not a pure compound. The molecular formula for water is h 2 o. Italian dressing is an example of. vinegar is not considered a compound, as it is a mixture of acetic acid and water that can be physically separated. So, there are actually two main chemical formulas involved. On the other hand, vinegar can also be. no, vinegar is not a pure compound. It is a mixture that includes substances other than acetic acid and water. vinegar consists of acetic acid (ch 3 cooh), water, and trace amounts of other chemicals, which may include flavorings. in this sense, vinegar can be considered a compound due to the acetic acid it contains. a mixture with a composition that varies from point to point is called a heterogeneous mixture. if a substance can be separated into its elements, it is a compound. If its composition is uniform throughout, it is a homogeneous mixture. The concentration of the acetic acid is variable. If a substance is not chemically pure, it is either a heterogeneous mixture or a homogeneous mixture. The structural formula for acetic acid is ch 3 cooh.
From circuitdiagramalexandra.z5.web.core.windows.net
Element Compound Mixture Diagram Is Vinegar An Element A Compound Or A Mixture If its composition is uniform throughout, it is a homogeneous mixture. a mixture with a composition that varies from point to point is called a heterogeneous mixture. in this sense, vinegar can be considered a compound due to the acetic acid it contains. vinegar consists of acetic acid (ch 3 cooh), water, and trace amounts of other. Is Vinegar An Element A Compound Or A Mixture.
From diagrampartforgivably.z13.web.core.windows.net
Homogeneous Mixture Particle Diagram Is Vinegar An Element A Compound Or A Mixture Italian dressing is an example of. vinegar is not considered a compound, as it is a mixture of acetic acid and water that can be physically separated. The structural formula for acetic acid is ch 3 cooh. vinegar consists of acetic acid (ch 3 cooh), water, and trace amounts of other chemicals, which may include flavorings. in. Is Vinegar An Element A Compound Or A Mixture.
From www.youtube.com
Pure Substances, Elements, Compounds, Homogenous & Heterogenous Mixture Is Vinegar An Element A Compound Or A Mixture The concentration of the acetic acid is variable. vinegar consists of acetic acid (ch 3 cooh), water, and trace amounts of other chemicals, which may include flavorings. if a substance can be separated into its elements, it is a compound. On the other hand, vinegar can also be. The molecular formula for water is h 2 o. The. Is Vinegar An Element A Compound Or A Mixture.
From mungfali.com
Identifying Elements Compounds And Mixtures Is Vinegar An Element A Compound Or A Mixture a mixture with a composition that varies from point to point is called a heterogeneous mixture. If its composition is uniform throughout, it is a homogeneous mixture. if a substance can be separated into its elements, it is a compound. The concentration of the acetic acid is variable. So, there are actually two main chemical formulas involved. . Is Vinegar An Element A Compound Or A Mixture.
From www.youtube.com
Chemical Formula for Vinegar (Acetic Acid or Ethanoic Acid) YouTube Is Vinegar An Element A Compound Or A Mixture If its composition is uniform throughout, it is a homogeneous mixture. vinegar is not considered a compound, as it is a mixture of acetic acid and water that can be physically separated. if a substance can be separated into its elements, it is a compound. If a substance is not chemically pure, it is either a heterogeneous mixture. Is Vinegar An Element A Compound Or A Mixture.
From armanitaromartin.blogspot.com
Difference Between Mixture and Compound ArmanitaroMartin Is Vinegar An Element A Compound Or A Mixture It is a mixture that includes substances other than acetic acid and water. If its composition is uniform throughout, it is a homogeneous mixture. On the other hand, vinegar can also be. The molecular formula for water is h 2 o. in this sense, vinegar can be considered a compound due to the acetic acid it contains. vinegar. Is Vinegar An Element A Compound Or A Mixture.
From learningschooldodirniuo.z4.web.core.windows.net
Mixture Vs Substance Examples Is Vinegar An Element A Compound Or A Mixture The structural formula for acetic acid is ch 3 cooh. The molecular formula for water is h 2 o. If a substance is not chemically pure, it is either a heterogeneous mixture or a homogeneous mixture. if a substance can be separated into its elements, it is a compound. On the other hand, vinegar can also be. It is. Is Vinegar An Element A Compound Or A Mixture.
From dxokeqoie.blob.core.windows.net
What Is The Chemical Formula For Vinegar at Mary Farr blog Is Vinegar An Element A Compound Or A Mixture The molecular formula for water is h 2 o. vinegar consists of acetic acid (ch 3 cooh), water, and trace amounts of other chemicals, which may include flavorings. So, there are actually two main chemical formulas involved. If its composition is uniform throughout, it is a homogeneous mixture. Italian dressing is an example of. a mixture with a. Is Vinegar An Element A Compound Or A Mixture.
From mavink.com
Venn Diagram Elements Compounds And Mixtures Is Vinegar An Element A Compound Or A Mixture vinegar consists of acetic acid (ch 3 cooh), water, and trace amounts of other chemicals, which may include flavorings. So, there are actually two main chemical formulas involved. a mixture with a composition that varies from point to point is called a heterogeneous mixture. if a substance can be separated into its elements, it is a compound.. Is Vinegar An Element A Compound Or A Mixture.
From vinegarorbleach.weebly.com
Vinegar Lewis Structure By serene falzone Is Vinegar An Element A Compound Or A Mixture The structural formula for acetic acid is ch 3 cooh. The molecular formula for water is h 2 o. So, there are actually two main chemical formulas involved. If a substance is not chemically pure, it is either a heterogeneous mixture or a homogeneous mixture. Italian dressing is an example of. vinegar is not considered a compound, as it. Is Vinegar An Element A Compound Or A Mixture.
From www.slideserve.com
PPT Matter and Change PowerPoint Presentation, free download ID2199820 Is Vinegar An Element A Compound Or A Mixture Italian dressing is an example of. vinegar consists of acetic acid (ch 3 cooh), water, and trace amounts of other chemicals, which may include flavorings. in this sense, vinegar can be considered a compound due to the acetic acid it contains. The structural formula for acetic acid is ch 3 cooh. If its composition is uniform throughout, it. Is Vinegar An Element A Compound Or A Mixture.
From www.gauthmath.com
Solved The diagram shows the displayed formula of a substance. Is this Is Vinegar An Element A Compound Or A Mixture So, there are actually two main chemical formulas involved. The molecular formula for water is h 2 o. On the other hand, vinegar can also be. a mixture with a composition that varies from point to point is called a heterogeneous mixture. The structural formula for acetic acid is ch 3 cooh. vinegar consists of acetic acid (ch. Is Vinegar An Element A Compound Or A Mixture.
From slideplayer.com
Elements, Compounds & Mixtures! Mr. Coffey. ppt download Is Vinegar An Element A Compound Or A Mixture It is a mixture that includes substances other than acetic acid and water. Italian dressing is an example of. no, vinegar is not a pure compound. vinegar consists of acetic acid (ch 3 cooh), water, and trace amounts of other chemicals, which may include flavorings. vinegar is not considered a compound, as it is a mixture of. Is Vinegar An Element A Compound Or A Mixture.
From manualworshipped.z14.web.core.windows.net
Diagram Of Mixture Of Elements Is Vinegar An Element A Compound Or A Mixture vinegar consists of acetic acid (ch 3 cooh), water, and trace amounts of other chemicals, which may include flavorings. So, there are actually two main chemical formulas involved. Italian dressing is an example of. vinegar is not considered a compound, as it is a mixture of acetic acid and water that can be physically separated. If a substance. Is Vinegar An Element A Compound Or A Mixture.
From printablelistquinta.z21.web.core.windows.net
Homogeneous And Heterogeneous Lesson Plan Is Vinegar An Element A Compound Or A Mixture The concentration of the acetic acid is variable. Italian dressing is an example of. If a substance is not chemically pure, it is either a heterogeneous mixture or a homogeneous mixture. no, vinegar is not a pure compound. If its composition is uniform throughout, it is a homogeneous mixture. if a substance can be separated into its elements,. Is Vinegar An Element A Compound Or A Mixture.
From enginedbscientizes.z21.web.core.windows.net
Mixture Of Elements And Compounds Diagram Is Vinegar An Element A Compound Or A Mixture The molecular formula for water is h 2 o. The structural formula for acetic acid is ch 3 cooh. If its composition is uniform throughout, it is a homogeneous mixture. On the other hand, vinegar can also be. no, vinegar is not a pure compound. Italian dressing is an example of. a mixture with a composition that varies. Is Vinegar An Element A Compound Or A Mixture.
From mavink.com
Venn Diagram Elements Compounds And Mixtures Is Vinegar An Element A Compound Or A Mixture If its composition is uniform throughout, it is a homogeneous mixture. vinegar is not considered a compound, as it is a mixture of acetic acid and water that can be physically separated. The molecular formula for water is h 2 o. a mixture with a composition that varies from point to point is called a heterogeneous mixture. . Is Vinegar An Element A Compound Or A Mixture.
From quizlet.com
Elements, Compounds, and Mixtures Diagram Quizlet Is Vinegar An Element A Compound Or A Mixture The structural formula for acetic acid is ch 3 cooh. It is a mixture that includes substances other than acetic acid and water. The molecular formula for water is h 2 o. Italian dressing is an example of. a mixture with a composition that varies from point to point is called a heterogeneous mixture. So, there are actually two. Is Vinegar An Element A Compound Or A Mixture.